Introduction: Classical Hodgkin lymphoma (cHL) is characterized by genetic alterations in 9p24.1, leading to overexpression of PD-L1 ligand. GLS-010 is a novel fully human anti-PD-1 monoclonal antibody (mAb) and exhibited favorable results in previous Phase I study. The aim of this study was to evaluate the safety and efficacy profile of GLS-010 in Chinese patients (pts) with relapsed or refractory cHL.
Methods: In this multi-center, single-arm Phase II clinical trial, pts with relapsed or refractory cHL after at least 2 lines of prior systemic chemotherapies were enrolled and treated with GLS-010 240mg every 2 weeks until disease progression, death, unacceptable toxicity or withdraw from the study. Efficacy was assessed with the primary endpoint of objective response rate (ORR) by independent review committee (IRC) per Lugano 2014. Adverse events (AEs) were graded by NCI CTCAE v4.03.
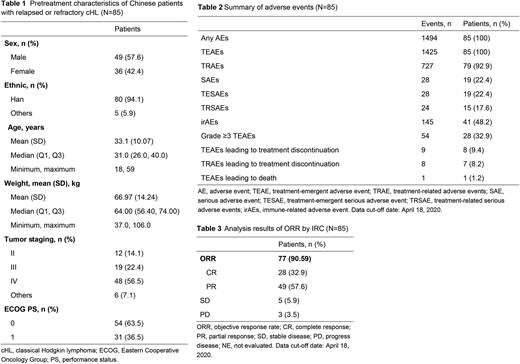
Results: As of April 18, 2020, a total of 85 pts with relapsed or refractory cHL received a median of 14.5 treatment cycles (1 cycle include 2 injections). The pretreatment characteristics of the pts are shown in Table 1. At a median follow-up of 15.8 months, 28.2% (24/85) of pts discontinued treatment. As shown in Table 2, treatment-related adverse events (TRAEs) of any grade occurred in 79 (92.9%) of 85 patients, most of which were Grade 1-2. The most common TRAEs were fever (27/85, 31.8%), neutrophil count decreased (17/85, 20%) and alanine aminotransferase (ALT) increased (17/85, 20%). Grade ≥3 TRAEs occurred in 25 (29.4%) pts, most commonly, hepatic function abnormal (5/85, 5.88%) and hyperuricaemia (4/85, 4.71%). For all the 85 pts, ORR was 90.59% (77/85, 95%CI: 82.30-95.85) with 32.9% (28/85) of patients achieving a CR and 57.6% (49/85) of patients achieving a PR (Table 3). Median duration of response (DoR) and progression free survival (PFS) were not reached yet.
Conclusions: GLS-010 showed impressive anti-tumor activity (ORR=90.59%) and manageable safety profile in Chinese patients with relapsed or refractory cHL, and could be a new safe and effective treatment option.
Meng:Guangzhou Gloria Biosciences Co., Ltd.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal